Individual-and pair-based models of epidemic spreading: Master equations and analysis of their forecasting capabilities
Abstract
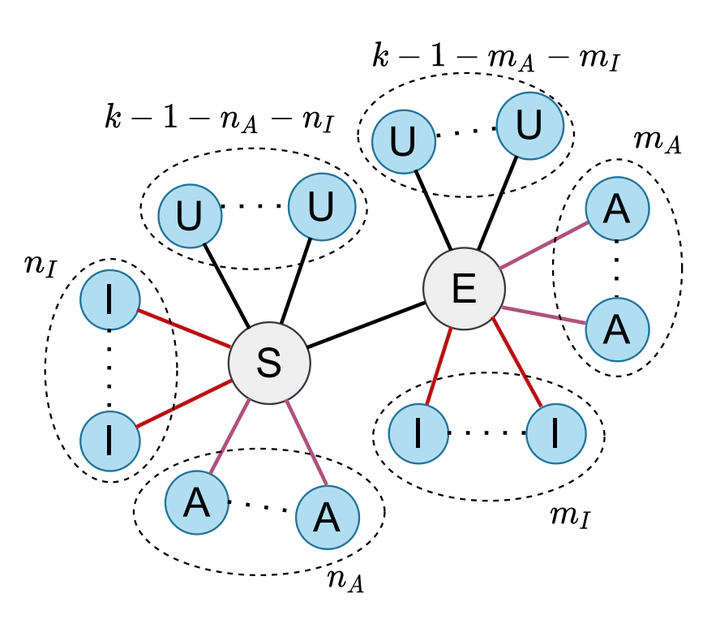
Epidemic models are crucial to understand how an infectious disease spreads in a population and to devise the best containment strategies. Compartmental models can provide a fine-grained description of the evolution of an epidemic when microscopic information on the network of contacts among individuals is available. However, coarser-grained descriptions prove also to be useful in many aspects. They allow to derive closed expressions for key parameters, such as the basic reproduction number, to understand the relationship between the model parameters, and also to derive fast and reliable predictions of macroscopic observables for a disease outbreak. The so-called population models can be developed at different levels of coarse-graining, so it is crucial to determine (i) to which extent and how the existing correlations in the contact network have to be included in these models and (ii) what is their impact on the model ability to reproduce and predict the time evolution of the populations at the various stage of the disease. In this work, we address these questions through a systematic analysis of two discrete-time SEAIR (susceptible-exposed-asymptomatic-infected-recovered) population models, the first one developed assuming statistical independence at the level of individuals, and the other one assuming independence at the level of pairs. We provide a detailed derivation and analysis of both models, focusing on their capability to reproduce an epidemic process on different synthetic networks, and then comparing their predictions under scenarios of increasing complexity. We find that, although both models can fit the time evolution of the compartment populations obtained through microscopic simulations, the epidemic parameters adopted by the individual-based model for this purpose may significantly differ from those of the microscopic simulations. However, pair-based model provides not only more reliable predictions of the dynamical evolution of the variables but also a good estimation of the epidemic parameters. The difference between the two models is even more evident in the particularly challenging scenario when one or more variables are not measurable, and therefore are not available for model tuning. This occurs for instance with asymptomatic infectious individuals in the case of COVID-19, an issue that has become extremely relevant during the recent pandemic. Under these conditions, the pairwise model again proves to perform much better than the individual-based representation, provided that it is fed with adequate information which, for instance, to be collected, may require a more detailed contact tracing. Overall, our results thus hallmark the importance of acquiring the proper empirical data to fully unfold the potentialities of models incorporating more sophisticated assumptions on the correlations among nodes in the contact network.